Summary
This week we focused heavily on the mechanics of cells. This included how cells “eat” and “drink,” as well as what makes up the borders of cells and how materials flow through and across these boundaries, aka cell membranes.
Terms
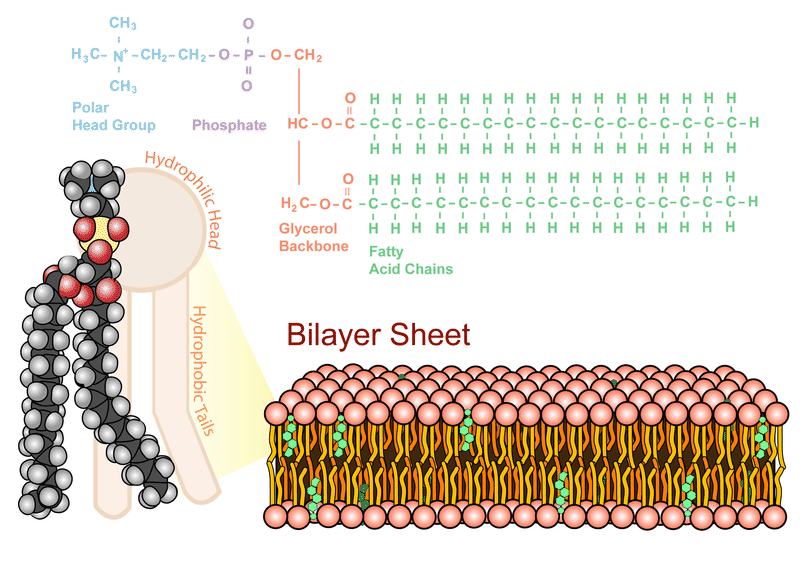
Phospholipid Bilayer– the backbone of the cell membrane, making up most of its surface and structure. It is comprised of two layers of lipids aligned with their hydrophobic fatty acid tails facing each other and their hydrophilic heads facing each other. This provides an effect boundary for the cell, through which only small nonpolar molecules can pass without the help of proteins.
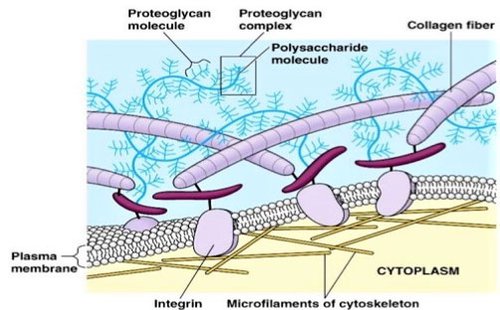
Extra Cellular Matrix- Wire like in appearance, strands of proteins that attach to cells and hold them to each other.
Passive Transport- The action of molecules moving across the bilayer with no effort done by the cell. This happens when there are varying concentrations of molecules inside vs outside the bilayer, so molecules flow on their own from high concentration areas to low concentration areas.

Active Transport- The action of cells using ATP and protein pumps to ship molecules across the membrane, especially from low concentration to high concentration.
Tonicity- A relative measurement of the combined concentration of all solutes in solution.
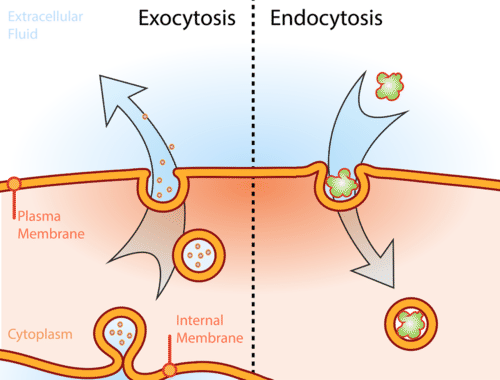
Endocytosis- The process by which a cell will “eat” a mass of food (or something like a plastid or mitochondria) by wrapping its cell membrane around the item then enveloping it inside the cell to be digested.
Exocytosis- The reverse of Endocytosis.
Questions Going Forward:
This past week has made me more excited to learn about specific cell mechanics, and to better understand how my own body functions. That being said, how do ribosomes actually work? Why don’t ribosomes read DNA instead of RNA? Can cells merge? How do cells recognize other cells as part of the same whole? How is food and minerals delivered to cells?