
This week we began learning some biochemistry, focusing on the properties of water. To start, we reviewed and defined the three subatomic parrticles we will need to know:
- protons: positively charged particles found in the atom’s nucleus. Have a mass of 1 amu and a charge of +1
- neutrons: neutrally charged nucleus particles, mass of 1 amu
- electrons: negatively charged particles that orbit the nucleus. Basically massless and have a charge or -1
These are all what makes up an atom. An element is a type of atom with a specific number of protons, and is the smallest unit of a substance that still retains its properties. (ex. carbon, 6 protons). There are about 120 different elements represented on the periodic table, of which biology uses CHONPS (carbon, hydrogen, oxygen, nitrogen, phosphorous, silicon) most of the time.
We also learned about how atoms interact, i.e. different types of atomic bonds.
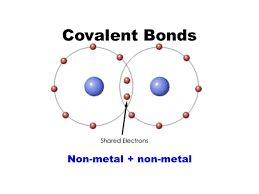
- Covalent Bonds- bonds created by atoms ‘sharing’ an electron. Ex. O=O bonds or C-H bonds. These bonds are relatively strong.
- Ionic bonds- created when one atom gives an electron to another atom. This usually happens to complete the outer shell(s) of one or both atoms. This creates an attraction between the atoms as one atom is now negatively charged while the other is positive. In general these bonds are relatively strong.
- Hydrogen Bonds- Intermolecular bonds that form between the positively charged hydrogen of a polar molecule and the partially negatively charged oxygen or nitrogen end of another polar molecule (ex. H — O bonds between water molecules)
Next, we learned about why water is so important to human bodies. The primary reason for this is that water has a high specific heat (4.184 J/g*C). This means that it takes a lot of energy to change the temperature of an organism containing lots of water, so it is easier for said organism to maintain homeostasis.
Next we had a discussion of the pH scale and what it measures. We read an interesting set of stories that explained how a lower pH (0-7) indicates an acidic solution, while a high pH (7-14) indicates a basic solution. An acid is a substance that donates protons to solution, like HCl, while a base is a substance that accepts protons (like bleach). The pH of a solution is the -log[H+].
Questions Going Forward
Will we need to memorize the body’s buffer compounds? How much chemistry will we need to know for the AP test? Will we do a lab regarding pH or specific heat?



